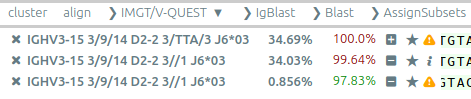
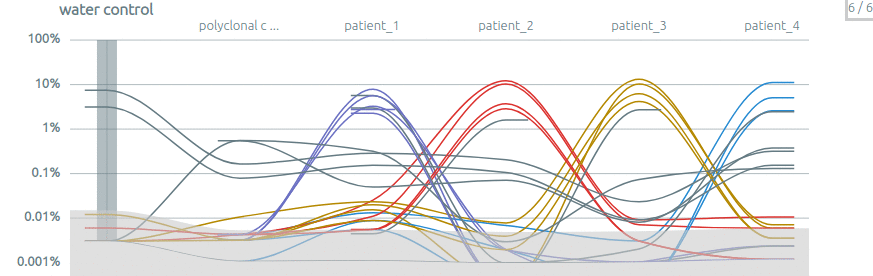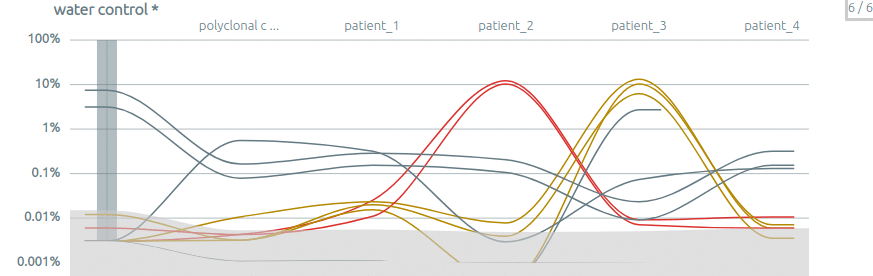
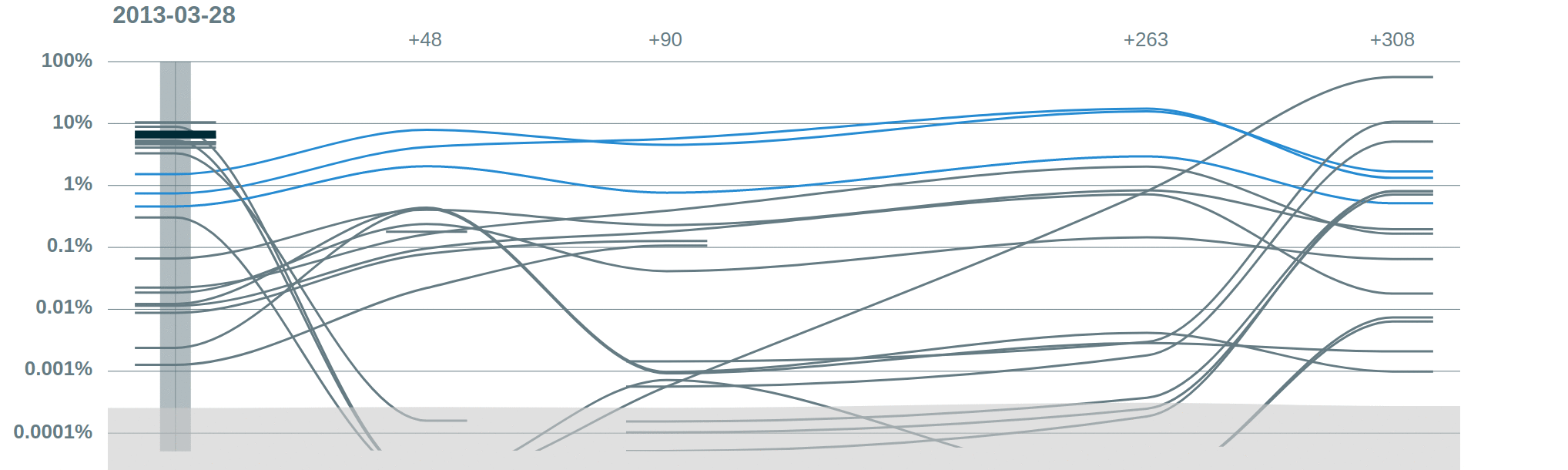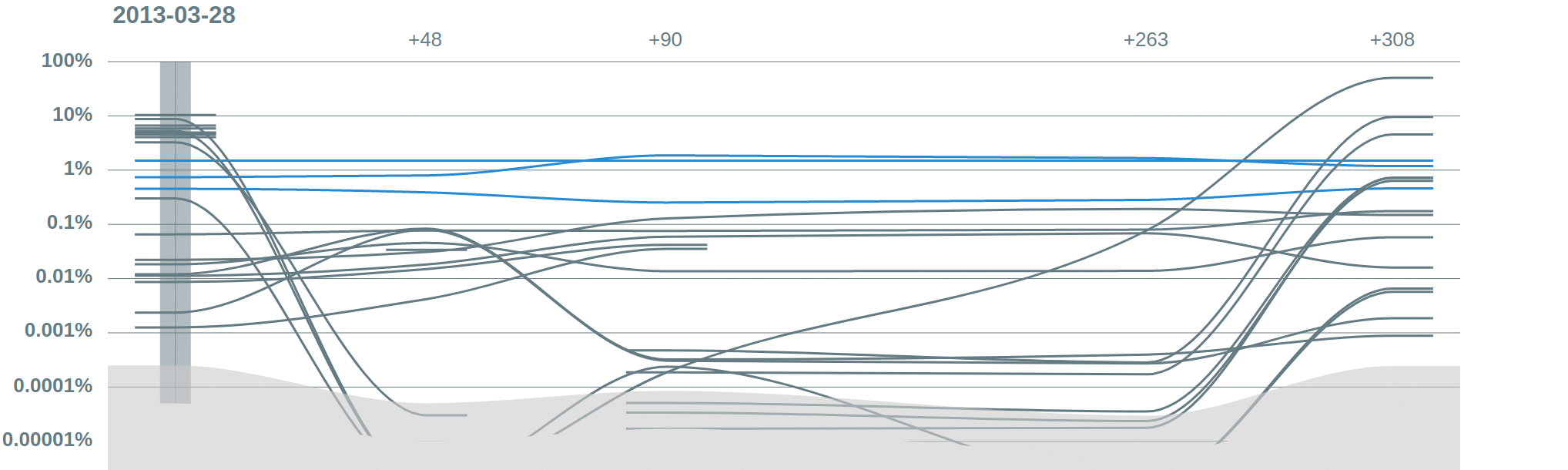
Some publications using Vidjil
Chrystelle Abdo et al., Caution encouraged in next-generation sequencing immunogenetic analyses in acute lymphoblastic leukemia, Blood, 2020, 136(9):1105–1107, https://doi.org/10.1182/blood.2020005613
Jean-Sebastien Allain et al., IGHV segment utilization in immunoglobulin gene rearrangement differentiates patients with anti-myelin-associated glycoprotein neuropathy from others immunoglobulin M-gammopathies, Haematologica, 2018, 103:e207-e210, http://dx.doi.org/10.3324/haematol.2017.177444
Kristian Assing et al., A Novel CDC42 Variant with Impaired Thymopoiesis, IL-7R Signaling, PAK1 Binding, and TCR Repertoire Diversity Journal of Clinical Immunology, 2023, https://doi.org/10.1007/s10875-023-01561-0
Jack Bartram et al., High throughput sequencing in acute lymphoblastic leukemia reveals clonal architecture of central nervous system and bone marrow compartments, Haematologica, 2018, https://dx.doi.org/10.3324%2Fhaematol.2017.174987
Sébastien Bender et al., Immunoglobulin variable domain high-throughput sequencing reveals specific novel mutational patterns in POEMS syndrome, Blood, 2020, https://doi.org/10.1182/blood.2019004197
Marie-Laure Boulland et al., Reliable IGHV status assessment by next generation sequencing in routine practice for chronic lymphocytic leukemia, Leukemia & Lymphoma, 2021, https://doi.org/10.1080/10428194.2021.1933476
Estelle Bourbon et al., Next-CLL: A New Next-Generation Sequencing–Based Method for Assessment of IGHV Gene Mutational Status in Chronic Lymphoid Leukemia, The Journal of Molecular Diagnostics, 2023, https://doi.org/10.1016/j.jmoldx.2023.01.009
Monika Brüggemann et al., on behalf of the EuroClonality-NGS working group, Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study, Leukemia, 2019, 33, 2241–2253, https://doi.org/10.1038/s41375-019-0496-7
Roberta Cavagna et al., Capture-based Next-Generation Sequencing Improves the Identification of Immunoglobulin/T-Cell Receptor Clonal Markers and Gene Mutations in Adult Acute Lymphoblastic Leukemia Patients Lacking Molecular Probes, Cancers, 2020, 12(6), 1505, https://doi.org/10.3390/cancers12061505
Rodolfo P. Correia et al., High‐throughput sequencing of immunoglobulin heavy chain for minimal residual disease detection in B‐lymphoblastic leukemia, Int. Journal of Laboratory Hematology, 2021, https://doi.org/10.1111/ijlh.13453
Frédéric Davi et al., on behalf of ERIC, the European Research Initiative on CLL, and the EuroClonality-NGS Working Group, Immunoglobulin gene analysis in chronic lymphocytic leukemia in the era of next generation sequencing, 2020 Leukemia, 2020, https://doi.org/10.1038/s41375-020-0923-9
Rachel Dobson et al., Widespread in situ follicular neoplasia in patients who subsequently developed follicular lymphoma, The Journal of Pathology, 2021, https://doi.org/10.1002/path.5861
Yann Ferret et al., Multi-loci diagnosis of acute lymphoblastic leukaemia with high-throughput sequencing and bioinformatics analysis, British Journal of Haematology, 2016, 173, 413–420, https://hal.archives-ouvertes.fr/hal-01279160
Henrike J. Fischer et al., Modulation of CNS autoimmune responses by CD8+ T cells coincides with their oligoclonal expansion, Journal of Neuroimmunology, 2015, S0165-5728(15)30065-5, http://dx.doi.org/10.1016/j.jneuroim.2015.10.020
Navarro Nilo Giusti et al., Test trial of spike-in immunoglobulin heavy-chain (IGH) controls for next generation sequencing quantification of minimal residual disease in acute lymphoblastic leukaemia, British Journal of Haematology, 2020, 189: e150-e154, https://doi.org/10.1111/bjh.16571
Isadoral Heraud et al., Monoclonal B-cell lymphocytosis with a non-CLL immunophenotype–Review of 34 cases Annales de Biologie Clinique, 2023, https://www.jle.com./fr/revues/abc/e-docs/monoclonal_b_cell_lymphocytosis_with_a_non_cll_immunophenotype_review_of_34_cases_330642/article.phtml?tab=texte
Irene Jo et al., Considerations for monitoring minimal residual disease using immunoglobulin clonality in patients with precursor B-cell lymphoblastic leukemia, Clinica Chimica Acta, 2019, https://doi.org/10.1016/j.cca.2018.10.037
Natalia Izotova et al., Long-term lymphoid progenitors independently sustain naïve T and NK cell production in humans, Nature Communications, 2021, https://doi.org/10.1038/s41467-021-21834-9
Vincent Jauvague et al., RNA-based immunoglobulin repertoire sequencing is a new tool for the management of monoclonal gammopathy of renal (kidney) significance. Kidney International, 2022, 101(2), 331-337, https://doi.org/10.1016/j.kint.2021.10.017
Takashi Kanamori et al., Genomic analysis of multiple myeloma using targeted capture sequencing in the Japanese cohort, British Journal of Haematology, 2020, https://doi.org/10.1111/bjh.16720
Kenji Kimura et al., Identification of Clonal Immunoglobulin λ Light-Chain Gene Rearrangements in AL Amyloidosis Using Next-Generation Sequencing, Experimental Hematology, 2021, 101:34-41.e4 https://doi.org/10.1016/j.exphem.2021.08.001
Michaela Kotrova et al., The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL, Blood, 2015, 126:1045-1047, http://dx.doi.org/10.1182/blood-2015-07-655159
Michaela Kotrova et al., Next‐generation amplicon TRB locus sequencing can overcome limitations of flow‐cytometric Vβ expression analysis and confirms clonality in all T‐cell ,prolymphocytic leukemia cases, Cytometry Part A, 93(11):1118-1124, 2018, http://dx.doi.org/10.1002/cyto.a.23604
Anton W. Langerak, High-Throughput Immunogenetics for Clinical and Research Applications in Immunohematology: Potential and Challenges, Journal of Immunology, 2017, 198(10):3765-3774, https://dx.doi.org/10.4049/jimmunol.1602050
Yannick Le Bris et al., Single Capture High Throughput Sequencing Assay for Combined V(D)J Clonality Analysis and Oncogene Mutations in the Diagnosis of T and B Lymphoid Malignancies, ASH 2021, Blood, 138(S1):2404, https://doi.org/10.1182/blood-2021-151083
Zhenhua Li et al., Identifying IGH disease clones for MRD monitoring in childhood B-cell acute lymphoblastic leukemia using RNA-Seq, Leukemia, 2020, 34:2418-2429, http://dx.doi.org/10.1038/s41375-020-0774-4
Ralf A. Linker et al., Thymocyte-derived BDNF influences T-cell maturation at the DN3/DN4 transition stage, European Journal of Immunology, 2015, 45, 1326-1338, http://dx.doi.org/10.1002/eji.201444985
Ming Liang Oon et al., T-Cell Lymphoma Clonality by Copy Number Variation Analysis of T-Cell Receptor Genes, Cancers, 2021, 13(2), 340, https://dx.doi.org/10.3390/cancers13020340
Alejandro Medina et al., Comparison of next-generation sequencing (NGS) and next-generation flow (NGF) for minimal residual disease (MRD) assessment in multiple myeloma, Blood Cancer Journal, 10, 108, 2020, https://doi.org/10.1038/s41408-020-00377-0
Dai Nishijima et al., Capture Sequencing Is a Useful Method for Comprehensive Clonality Analysis Based on Ig/TCR Gene Rearrangements in Acute Lymphoblastic Leukemia, ASH 2018, Blood, 132(S1):1543, https://doi.org/10.1182/blood-2018-99-115624
Alexis Piedrafita et al., Spectrum of Kidney Disorders Associated with T-Cell Immunoclones, Journal of Clinical Medicine, 2022, 11(3), 604, https://doi.org/10.3390/jcm11030604
Edit Porpaczy et al., Aggressive B-cell lymphomas in patients with myelofibrosis receiving JAK1/2 inhibitor therapy, Blood, 2018, https://dx.doi.org/10.1182/blood-2017-10-810739
Natalia Izotova et al., Long-term lymphoid progenitors independently sustain naïve T and NK cell production in humans, Nature Communications, 2021, 12:1622, https://doi.org/10.1038/s41467-021-21834-9
Rathana Kim et al., Adult T-cell acute lymphoblastic leukemias with IL7R pathway mutations are slow-responders who do not benefit from allogeneic stem-cell transplantation, Leukemia, 2020, 34, 1730-1740, https://dx.doi.org/10.1038/s41375-019-0685-4
Cédric Pastoret et al., Molecular mechanisms underlying transformation of large granular lymphocytic leukemia to high-grade T-cell lymphoma, Leukemia, 2023, https://www.nature.com/articles/s41375-023-01922-z
Mikaël Salson et al., High-throughput sequencing in acute lymphoblastic leukemia: Follow-up of minimal residual disease and emergence of new clones, Leukemia Research, 2017, 53, 1–7, http://dx.doi.org/10.1016/j.leukres.2016.11.009
Masashi Sanada et al., Targeted-Capture Sequencing Is a Useful Method for MRD Markers Screening in KMT2A (MLL) Rearranged Leukemia, ASH 2019, Blood, 134(S1):2759, https://doi.org/10.1182/blood-2019-125421
Florian Scherer et al., Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA, Science Translational Medicine, 2016, 8, 364ra155, http://dx.doi.org/10.1126/scitranslmed.aai8545
V. Seitz et al., Evidence for a role of RUNX1 as recombinase cofactor for TCRβ rearrangements and pathological deletions in ETV6-RUNX1 ALL Scientific Reports, 2020, 10:10024, https://doi.org/10.1038/s41598-020-65744-0
Udo zur Stadt et al., Characterization of novel, recurrent genomic rearrangements as sensitive MRD targets in childhood B-cell precursor ALL, Blood Cancer Journal, 2019, https://doi.org/10.1038/s41408-019-0257-x
Lucia Stranavova et al., Heterologous Cytomegalovirus and Allo-Reactivity by Shared T Cell Receptor Repertoire in Kidney Transplantation, Frontiers in Immunology, 2019, https://doi.org/10.3389/fimmu.2019.02549
Manuela Tosi et al., MRD-Based Therapeutic Decisions in Genetically Defined Subsets of Adolescents and Young Adult Philadelphia-Negative ALL Cancers 2021, 13(9), 2108, https://doi.org/10.3390/cancers13092108
Amelie Trinquand et al., Toward Pediatric T Lymphoblastic Lymphoma Stratification Based on Minimal Disseminated Disease and NOTCH1/FBXW7 Status, HemaSphere, 2021, https://doi.org/10.1097/hs9.0000000000000641
Christine Wennerås et al., Infection with Neoehrlichia mikurensis promotes the development of malignant B-cell lymphomas , British Journal of Haematology, 2023, https://doi.org/10.1111/bjh.18652
Gary Wright et al., Clinical benefit of a high‐throughput sequencing approach for minimal residual disease in acute lymphoblastic leukemia, Pediatric Blood & Cancer, 2019, https://doi.org/10.1002/pbc.27787
Wen‐Qing Yao et al., Angioimmunoblastic T‐cell lymphoma contains multiple clonal T‐cell populations derived from a common TET2 mutant progenitor cell, The Journal of Pathology, 2019, https://doi.org/10.1002/path.5376
Yasuda et al., Clinical utility of target capture‐based panel sequencing in hematological malignancies: A multicenter feasibility study, Cancer Science, 2020, 111(9):3367-3378, https://dx.doi.org/10.1111/cas.14552
![]()